Temperature and dissolved oxygen (DO) are two important aquatic ecological factors. When the water temperature increased, the solubility of oxygen decreased. Heat commonly accompanied hypoxia. Heat and hypoxia stresses could affect a series of physiological and biochemical responses and drive the expression of regulatory genes.
The hard clam Mercenaria mercenaria is a heat- and hypoxia-tolerant species, which is an ideal model for investigating the mechanisms underlying environmental adaptation. However, the transcriptional regulation in this robust species under multiple stresses remains poorly studied.
Recently, the research team led by Prof. ZHANG Tao of Institute of Oceanology, Chinese Academy of Sciences (IOCAS), has explored the transcriptional regulation mechanism of hard clam under heat, hypoxia and combined stress.
The study was published in Aquaculture.
Comparative transcriptomic analyses showed that protein folding was significantly enriched in common differentially expressed heat-induced genes. Heat stress can destroy protein structures, protein folding plays a vital role in protein homeostasis. Molecular chaperones including HSP90 and TCP1 exhibited high expression in the heat-stress groups. Molecular chaperones assist in protein folding to ensure that protein homeostasis maintained in cells.
Microtubule-related GO terms were significantly enriched in hypoxia-induced genes under different temperatures. Microtubules are very important in forming the cytoskeleton and maintaining the cell structure and motility. Kinesin and dynein-related genes were upregulated in the hypoxia groups compared to the control. Dynein-mediated microtubule motor and movement might help cytoskeletal reorganization and maintain cell structure in hard clams under hypoxia stress.
Protein processing in endoplasmic reticulum and ubiquitin-mediated proteolysis were activated under heat plus hypoxia stress. Hard clams might employ a strict quality control system to alleviate cytotoxicity and maintain cell homeostasis by ensuring proper protein folding, enhancing recognition of misfolded protein, and facilitating damaged protein degradation through ubiquitin-mediated proteolysis.
This work was supported by China National Key R & D Program, Tianjin Science and Technology Commission Project, Tianjin Agricultural Committee Project and China Agriculture Research System of MOF and MARA.
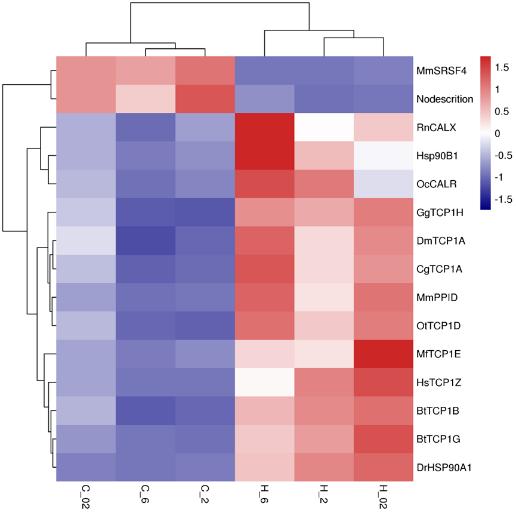
Fig. 1 Heat map of the expression analysis of 15 DEGs in GO: 0006457 (protein folding)
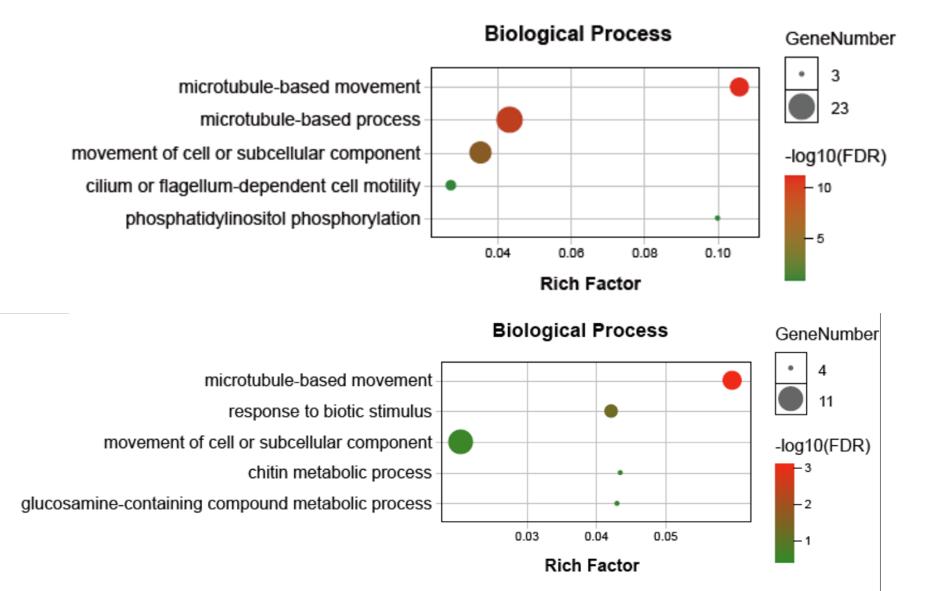
Fig. 2 Biological process enrichment results of common DEGs in response to hypoxia stress under normal and high temperature
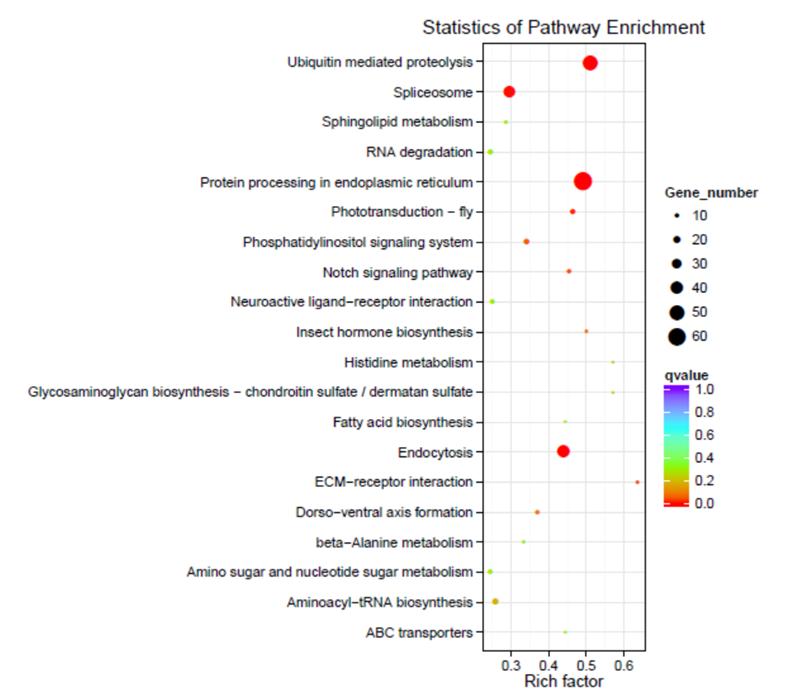
Fig. 3 KEGG enrichment results of common DEGs in response to heat plus hypoxia stress
Hu, Z., Feng, J., et al. (2021). Mechanisms of heat and hypoxia defense in hard clam: Insights from transcriptome analysis. Aquaculture, 737792.
HU Zhi
Institute of Oceanology
E-mail: huzhi@qdio.ac.cn
(Editor: ZHANG Yiyi)
|
|

Address: 7 Nanhai Road, Qingdao, Shandong 266071, China
Tel: 86-532-82898902 Fax: 86-532-82898612 E-mail: iocas@qdio.ac.cn


